Background:
Selinexor is an oral, selective inhibitor of nuclear export compound that blocks exportin 1 (XPO1), leading to retention of tumor suppressor proteins and apoptosis in malignant cells. Preclinical studies suggest synergistic activity when selinexor is combined with other treatments for multiple myeloma (MM). We hypothesize the addition of selinexor can recapture response to patients relapsing on their current carfilzomib (CFZ), pomalidomide (POM) or daratumumab (DARA)-based regimens.
Methods:
This is an ongoing phase 2b, open-label, multicenter investigator-initiated study to evaluate the safety and efficacy of selinexor plus CFZ, POM, or DARA. MM patients with documented disease progression or refractory disease while on current treatment with any CFZ-containing regimen (Arm 1), any POM-containing regimen (Arm 2), or any DARA-containing regimen (Arm 3) were eligible for this study. Patients were assigned to the respective arm only if their most recent line of therapy contained the specific drug of interest. Arm 1 consists of selinexor 80 mg orally on D1, 8, 15; CFZ 56 mg/m 2 IV on D1, 8, 15; and dexamethasone (DEX) 40 mg or 20 mg if ≥75 years old on D1, 8, 15, 22. Arm 2 consists of selinexor 60 mg orally on D1, 8, 15; POM 4 mg orally on D1-21; and DEX 40 mg or 20 mg if ≥75 years old on D1, 8, 15, 22. Arm 3 consists of selinexor 100 mg orally on D1, 8, 15, 22; DARA 16 mg/kg IV or 1,800 mg SQ dosing as per standard of care; and DEX 40 mg or 20 mg if ≥75 years old on D1, 8, 15, 22. The primary endpoint is overall response rate (ORR), which includes patients who experience partial response (PR), very good partial response (VGPR), complete response (CR), or stringent CR (sCR), based on International Myeloma Working Group response criteria (Kumar 2016). Secondary endpoints include duration of response (DOR), clinical benefit rate (CBR = sCR + CR + VGPR + PR + stable disease [SD]), progression-free survival (PFS), and treatment-emergent adverse events (TEAEs).
Results:
At data cutoff (July 14, 2023), there were 23 patients enrolled, including 20 patients on trial, 2 screen failures, and 1 in screening. Of the 20 patients on trial, 1 patient was enrolled onto Arm 1, 9 patients onto Arm 2, and 10 patients onto Arm 3. The median age was 66 years (range 52-83), 30% with ISS stage III MM, 75% with high-risk cytogenetics, 90% refractory to the last LOT, 50% triple refractory, and 35% quadruple refractory. Fifteen unique patients had high-risk cytogenetics, which included 9 patients with 1q21 duplication, 7 with t(4;14), 2 with del(17p)/monosomy 17, 4 with TP53 mutation, and 1 with complex karyotype.
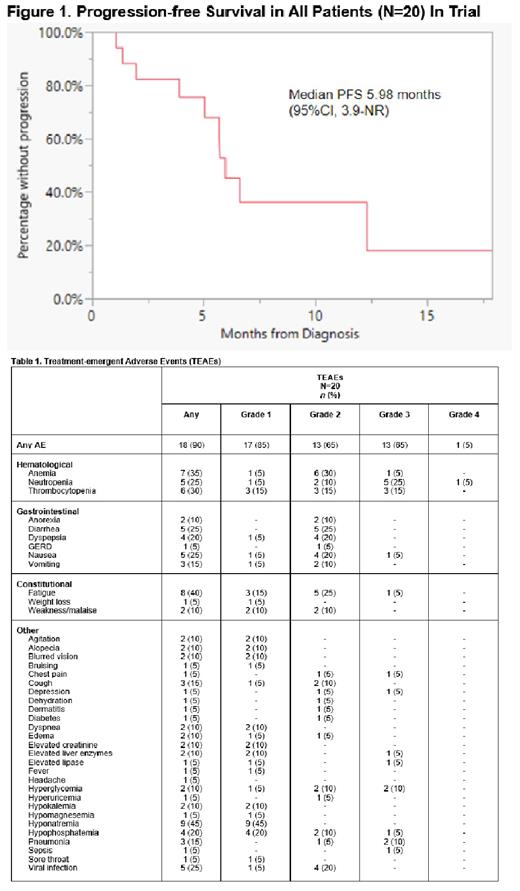
In 18 patients evaluable for response, the ORR was 33% (6/18; PR, 6 [33%]) and the CBR was 89% (16/18; PR, 6 [33%]; SD, 10 [56%]); 2 patients withdrew consent before response evaluation. For Arm 2, the ORR was 29% (2/7; PR, 2 [29%]) and the CBR was 86% (6/7; PR, 2 [29%]; SD, 4 [57%]). For Arm 3, the ORR was 44% (4/9; PR, 4 [44%]) and the CBR was 100% (9/9; PR, 4 [44%]; SD, 5 [56%]). In the overall cohort, median DOR was 9.46 months (95% CI, 5.1-12.3), and median PFS was 5.98 months (95% CI, 3.9-NR) (Figure 1).
There were 18/20 patients who experienced any grade TEAEs (Table 1). No treatment-related deaths occurred. The most common grade 3 TEAEs were neutropenia (25%), thrombocytopenia (15%), pneumonia (10%), and hyperglycemia (10%). One patient experienced a grade 4 TEAE of neutropenia. There were 3 unique patients who experienced SAEs related to study treatment, which included pneumonia (n=2), chest pain (n=1), sepsis (n=1), and lactic acidosis (n=1). Seventeen patients discontinued treatment due to disease progression (n=12) and withdrawal of consent (n=5). Two patients are deceased: one patient died during long-term follow-up unrelated to treatment of disease; and one patient was hospitalized for dehydration, anorexia, and hyponatremia after C1D8 of KCD then experienced progressive cytopenias upon discharge likely due to disease progression.
Conclusions:
Preliminary results suggest selinexor plus CFZ, POM, or DARA is safe and restores sensitivity to therapy in relapsed/refractory MM patients. In this population of heavily pretreated MM patients relapsing on their most recent line of therapy, the ORR of 33% and CBR 89% highlights the clinical potential and generally manageable side effect profile of selinexor as an add-on drug for patients with relapsed/refractory MM in combination with CFZ, POM, and DARA. Clinical trial information: NCT04661137.
Disclosures
Biran:Abbvie: Honoraria; Merck: Research Funding; Genomic Testing Cooperative: Divested equity in a private or publicly-traded company in the past 24 months; GSK: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Other: spouse of employee; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding. Parmar:Cellectar Biosciences: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. Feinman:BMS: Other: Spouse; Sanofi: Other: Spouse; Pepticom: Other: Spouse; COTA: Other: Spouse; Takeda: Other: Spouse; GSK: Other: Spouse; Karyopharm: Other: Spouse; Pfizer: Other: Spouse; Janssen Onocology: Other: Spouse; Menarini Silicon Biosystems: Other: Spouse; Neximmune: Other: Spouse. Siegel:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celularity Scientific: Consultancy, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal